2019-11-14

[technology] German scientists develop new electrolytes to put calcium batteries into use and replace lithium-ion batteries
According to foreign media reports, calcium based batteries are expected to achieve higher energy density at a lower manufacturing cost. Finally, this laboratory technology can replace the lithium-ion technology in the future energy storage system. However, the battery cannot be charged at room temperature by using the existing electrolyte. However, researchers at the Karlsruhe Institute of Technology (Kit) in Germany have developed a very promising electrolyte that makes rechargeable calcium batteries possible.
Now, researchers have successfully synthesized a new type of electrolyte, which is based on special organic calcium salts and can be charged at room temperature. Using this new electrolyte, researchers showed a calcium battery with high energy density, high energy storage capacity and fast charging.
The new electrolyte is an important basis for transforming experimental calcium batteries into practical calcium batteries. In electric vehicles, mobile electronic devices and fixed energy storage systems, such calcium batteries may one day replace the currently dominant lithium-ion batteries. (source: Gaishi automobile)
[market] the domestic hydrofluoric acid market price is temporarily stable
On November 12, the mainstream price of domestic anhydrous hydrofluoric acid manufacturers was 9000-9500 yuan / ton. The ex factory price of some manufacturers in the field was temporarily stable. The operating rate of domestic hydrofluoric acid manufacturers was general, the supply of goods in the field was sufficient, and the market price trend in the field remained low. (source: Business Club)
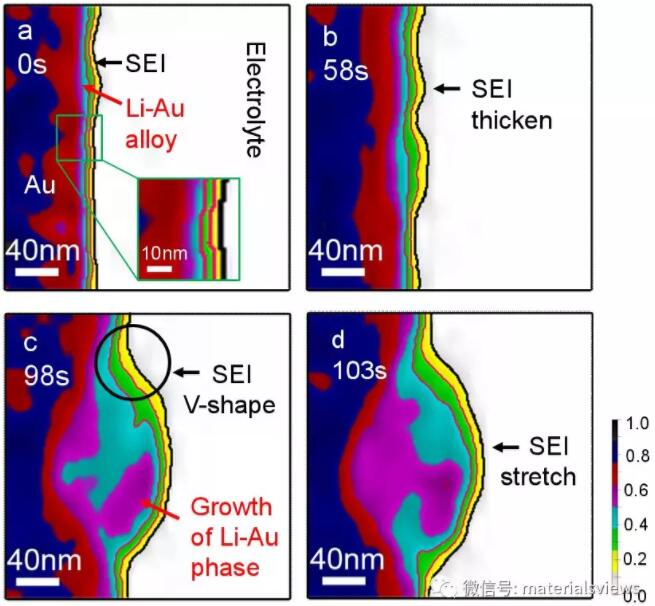
【technology】In situ dynamic observation of solid electrolyte phase interface facial mask (SEI) on the electrode surface of lithium battery by scanning transmission electron microscope
In the lithium metal battery system (including lithium-ion battery) with metal lithium as negative electrode, solid electrolyte phase interface facial mask (SEI) is a passive film spontaneously growing on the surface of the negative electrode, which plays an extremely important role in the dissolution and deposition of lithium ions during charge and discharge. The formation of SEI film mainly comes from the spontaneous reduction reaction of battery electrolyte on the electrode surface. It is an ion conduction and electronic insulating film. The dynamic process of its formation and fracture is closely related to the safety, capacity and cycle life of lithium metal battery. Because the instability of SEI film is the decisive factor leading to the nucleation and growth of lithium dendrites, which are often the culprit of battery short circuit and even explosion and ignition. Therefore, studying how to make SEI film stable and strong has become the top priority to realize the commercial application of lithium metal anode in the field of electric vehicles and other high energy density energy storage devices. Although the importance of in-depth study of SEI film is self-evident, because SEI film is directly grown on the surface of the negative electrode, its formation and stability are greatly affected by the volume change of the negative electrode in the process of charge and discharge and the electrochemical environment. Therefore, many key problems in SEI membrane structure and dynamic transformation process are still not very clear. This greatly limits the development of lithium ion battery and lithium metal battery technology.
Under the leadership of Professor Mingwei Chen of Johns Hopkins University in the United States, the international cooperation team of Shanghai Jiaotong University, Northeastern University of Japan and Johns Hopkins University in the United States used spherical aberration correction scanning transmission electron microscope technology to observe the formation, growth and decomposition process of solid electrolyte phase interface facial mask (SEI) in lithium-ion liquid batteries under high current and rapid charge and discharge conditions, The structure, growth and decomposition mechanism of SEI membrane in complex electrochemical environment were revealed. Different from the previously reported submicron phase contrast in-situ transmission electron microscope observation, Hou Chen, a doctoral student in the team, used high-angle annular dark field imaging (HAADF-STEM) and annular bright field imaging (abf-stem) in-situ scanning transmission electron microscopy with quality contrast to observe in real time the structural and morphological changes of the sub nanometer SEI film on the surface of the gold cathode in liquid lithium-ion batteries with the rapid insertion and removal of lithium ions. In the high-resolution mass contrast image, the layered structure of SEI film is clearly displayed: Green inorganic inner layer and yellow organic outer layer, as shown in the figure. In situ observation further revealed that during the charging process, the growth of SEI film first formed an uneven sub nano microporous double-layer structure. With the charging process, SEI film gradually became uniform, and the inner inorganic layer also became dense from porous, while the outer organic layer still maintained the sub nano microporous structure. With the further growth of SEI film, when its film thickness exceeds the electron tunneling conduction distance, researchers found that the growth of SEI film changes from the decomposition of electrolyte directly on the electrode surface to the auxiliary growth of atomic groups in electrolyte. This mechanism is consistent with the first principle calculation. The destruction process of SEI film during discharge was also completely recorded by researchers. Through detailed structural analysis, researchers found that the coarse electrode surface and surface thorns formed by uneven shrinkage of electrode volume during lithium removal will accelerate the rupture of SEI film. The destruction of SEI membrane is mainly caused by the rapid dissolution of inorganic layer after contacting with electrolyte.
Professor Chen Mingwei and his team believe that this study provides a valuable experimental basis for understanding the basic dynamics of SEI films and a new idea for developing lithium electrodes with stable SEI films. It also provides necessary experimental support for the commercial application of lithium metal anode in lithium ion batteries and lithium metal batteries in the future. (source: materialsviews)
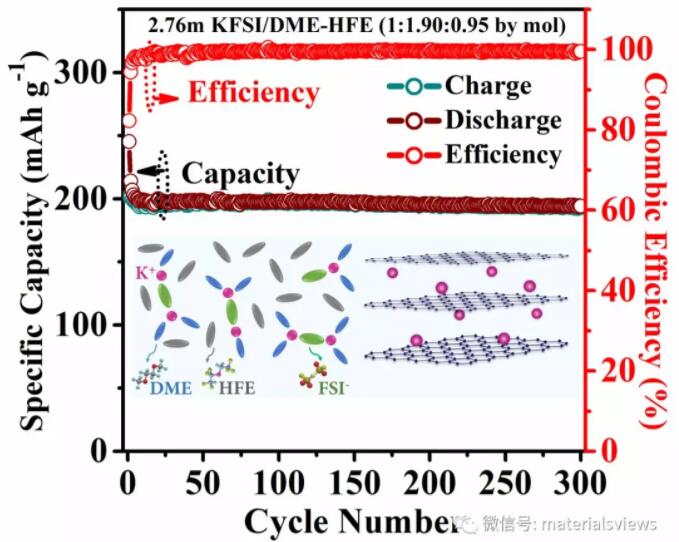
【technology】Aenm: local high concentration electrolyte helps improve the potassium storage performance of graphite anode for potassium ion batteries
In recent years, high concentration electrolytes (HCEs) system has been applied to the research of graphite anode. As the salt concentration in HCEs system increases, the number of free solvent molecules decreases significantly, so the voltage window of electrolyte is further widened. Different from traditional low concentration electrolytes (LCEs), solvent molecules are mainly involved in the formation mechanism of SEI. The SEI component on the negative surface of HCEs system mainly comes from the decomposition products of salt anions. Previously, it has been reported that using high concentration potassium difluorosulfimide (kfsi) system to improve the stability of SEI on the negative surface of potassium ion batteries, but the high cost and low electrode / diaphragm wettability of HCEs system still limit its further application.
In view of this, the research group of Professor wuyiying of Ohio State University in the United States and the team of Professor zhaidengyun of Shenzhen Graduate School of Tsinghua University have proposed a new solution, that is, adding a co solvent that can be miscible with the electrolyte solvent but does not dissolve salt itself into the HCEs system to form a localized high concentration electrolytes (lhces) with low salt concentration locally. By constructing lhces system, the original interconnected K solvated shell with three-dimensional structure can be broken without destroying a single K The high coordination environment of HCEs can also effectively avoid a series of problems caused by the use of HCEs. In this paper, the electrolyte system of lhces was constructed with kfsi as potassium salt, ethylene glycol dimethyl ether (DME) as solvent and 2,2,2-trifluoroethyl-1,1,2,2-tetrafluoroethyl ether (HFE) as cosolvent.
In this paper, the concept of lhces is extended to potassium ion battery system for the first time. After adding HFE, lhces can form locally highly coordinated K -DME solvates and SEI rich in potassium fluoride (KF) can effectively inhibit the solvent co intercalation of conventional ether electrolytes in graphite anode, and maintain the structural stability of graphite in the process of potassium ion de intercalation; At the same time, due to its high ionic conductivity (13.6mscm-1)And good electrode / diaphragm wettability, making the high load of commercial graphite (8mgcm-2)It shows a high specific capacity (it can still maintain ~200mahg after 300 cycles-1)。 In addition, the lhces constructed in this paper has a stable high voltage window (5.3vvs.k /K) , which can perfectly match the high-voltage Prussian blue cathode, and its non flammable characteristics are expected to significantly improve the safety performance of the battery. In particular, through the kinetic analysis of the process of potassium ion removal from graphite, this paper points out that the reaction rate of potassium ion insertion into graphite lamella to form low-order graphite intercalation compounds is slow, while the reaction kinetics of potassium ion removal is significantly improved.
In this paper, in order to improve the electrochemical potassium storage performance of commercial high load graphite anode, from the perspective of the protection of graphite electrolyte interface, the stability of SEI on the surface of graphite anode is effectively controlled by designing electrolyte components and solvation structure. Compared with traditional carbonate electrolyte, this system can significantly improve the electrochemical performance of graphite anode. The researchers believe that this study will provide a new idea for the study of the electrolyte system of graphite based potassium ion batteries. (source: materialsviews)
[think tank circle]The price of electrolyte basically capped
Recently, the electrolyte industry is relatively calm, with high market concentration, and there is basically no news of new projects being put into production. In addition, affected by the national safety and environmental protection policies and the rise in the price of raw materials such as solvents, the price of electrolyte has increased. However, due to the current stagnation in the installed capacity of power batteries, it is difficult to increase the demand. The industry expects that the price of electrolyte is basically capped, and it is difficult to rise significantly in the short term.
Article source:Battery net
0755-89480969
info@powercome.hk
B1202, building 1, Mogen Fashion Industrial Park, No. 10, shilongzi Road, Xinshi community, Dalang street, Longhua District, Shenzhen
www.powercome.hk