2019-09-06
Source: China Battery Alliance
Lithium ion batteryWith its high specific energy and power density, long cycle life, environment-friendly and other characteristics, in consumer electronicselectric vehicleandEnergy storageAnd other fields have been widely used. As the power source of new energy vehicles, lithium-ion batteries still have many problems in practical applications. For example, the energy density is significantly reduced under low temperature conditions, and the cycle life is correspondingly affected, which also seriously limits the scale use of lithium-ion batteries.

At present, researchers still argue about the main factors that cause the poor low-temperature performance of lithium-ion batteries, but the reasons are as follows:
1.. At low temperature, the viscosity of electrolyte increases and the conductivity decreases;
2. The electrolyte / electrode interface facial mask impedance and charge transfer impedance increase;
3. The migration rate of lithium ion in the active substance body decreases As a result, the polarization of electrode is intensified and the charge discharge capacity is reduced at low temperature.
In addition, in the process of low-temperature charging, especially in the case of low-temperature high-power charging, lithium metal precipitation and deposition will occur on the negative electrode, and the deposited metal lithium is easy to react irreversibly with the electrolyte, consuming a large amount of electrolyte. At the same time, the thickness of SEI film will further increase, resulting in the further increase of the impedance of the negative facial mask of the battery, and the re enhancement of battery polarization, which will greatly damage the low-temperature performance, cycle life and safety performance of the battery.
This paper summarizes the research progress of low-temperature performance of lithium-ion batteries, and systematically analyzes the main limiting factors of low-temperature performance of lithium-ion batteries. The modification methods of improving the low-temperature performance of battery by researchers in recent years are discussed from three aspects: positive electrode, electrolyte and negative electrode.
1、 Cathode material
Cathode material is one of the key materials for manufacturing lithium-ion batteries. Its performance directly affects the indicators of the battery, and the structure of the material has an important impact on the low-temperature performance of lithium-ion batteries.
LiFePO4 with olivine structure has the advantages of high specific discharge capacity, stable discharge platform, stable structure, excellent cycle performance and rich raw materials. It is the mainstream cathode material for lithium-ion power batteries. However, lithium iron phosphate belongs to the Pnma space group, P occupies the tetrahedral position, and the transition metal M occupies the octahedral position. The Li atom forms a migration channel along the one-dimensional direction of the [010] axis. This one-dimensional ion channel leads to the orderly separation or insertion of lithium ions in a single way, which seriously affects the diffusion ability of lithium ions in this material. Especially at low temperature, the diffusion of lithium ions in the body is further blocked, resulting in increased impedance, more serious polarization and poor low-temperature performance.
Nickel cobalt manganese based linixcoymn1-x-yo2 is a new kind of solid solution material developed in recent years, which has similar properties to LiCoO2 α- Nafeo2 single-phase layered structure. The material has the important advantages of high reversible specific capacity, good cycle stability, moderate cost and so on. It has also been successfully applied in the field of power batteries, and the application scale has developed rapidly. However, there are also some problems that need to be solved urgently, such as low electronic conductivity and poor stability of large magnification, especially with the increase of nickel content, the high and low temperature properties of materials become worse.
Lithium rich manganese based cathode materials have higher specific discharge capacity and are expected to become the cathode materials of the next generation of lithium-ion batteries. However, there are many problems in the practical application of lithium rich manganese base: the first irreversible capacity is high, and it is easy to change from layered structure to spinel structure in the process of charge and discharge, which makes the diffusion channel of Li blocked by the migrated transition metal ions, resulting in serious capacity attenuation, and poor ionic and electronic conductivity, resulting in poor magnification performance and low temperature performance.
The main ways to improve the ion diffusion performance of cathode materials at low temperature are:
1. The method of coating the active material body with materials with excellent conductivity improves the conductivity of the positive material interface, reduces the interface impedance, reduces the side reactions between the positive material and electrolyte, and stabilizes the material structure.
Rui et al. Studied the low temperature performance of carbon coated LiFePO4 by using cyclic voltammetry and AC impedance method. It was found that the discharge capacity gradually decreased with the decrease of temperature, and the capacity at -20 ° C was only 33% of the capacity at room temperature. The author believes that as the temperature decreases, the charge transfer impedance and Weber impedance in the battery gradually increase, and the difference of redox potential in the CV Curve increases, which indicates that the diffusion of lithium ions in the material slows down at low temperature, and the Faraday reaction kinetic rate of the battery weakens, resulting in a significant increase in polarization (Fig. 1).
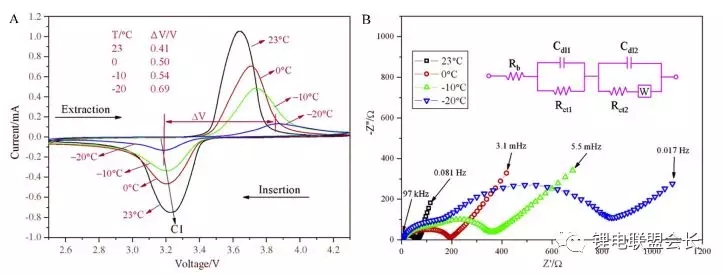
Fig. 1 CV (a) and EIS (b) curves of lfp/c at different temperatures
LV et al. Designed and synthesized a composite cathode material with fast ion conductor coated with lithium nickel cobalt manganate, which shows superior low-temperature performance and magnification performance, and still maintains a reversible capacity of 127.7mah · g-1 at -20 ° C, which is much better than 86.4mah · g-1 of lithium nickel cobalt manganate. The introduction of fast ion conductor with excellent ionic conductivity can effectively improve the diffusion rate of Li, which provides a new idea for improving the low-temperature performance of lithium-ion batteries.
2. The bulk phase of the material is doped by Mn, Al, Cr, Mg, F and other elements, and the layer spacing of the material is increased to improve the diffusion rate of Li in the bulk, reduce the diffusion impedance of Li, and then improve the low-temperature performance of the battery.
Zeng et al. Used Mn doping to prepare carbon coated LiFePO4 cathode material. Compared with the original LiFePO4, its polarization at different temperatures is reduced to a certain extent, significantly improving the electrochemical performance of the material at low temperature. Li et al. Doped lini0.5co0.2mn0.3o2 material with Al, and found that Al increased the layer spacing of the material, reduced the diffusion impedance of lithium ions in the material, and greatly improved its gram capacity at low temperature.
The phase transition of lithium iron phosphate cathode material from lithium iron phosphate phase to lithium iron phosphate phase in the charging process is slower than that from iron phosphate phase to lithium iron phosphate phase in the discharge process, and Cr doping can promote the phase transition from iron phosphate phase to lithium iron phosphate phase in the discharge process, so as to improve the rate performance and low temperature performance of LiFePO4.
3. Reduce the particle size of the material and shorten the Li migration path. It should be pointed out that this method will increase the specific surface area of the material and increase the side reactions with the electrolyte.
Zhao et al. Studied the effect of particle size on the low temperature performance of carbon coated LiFePO4 material, and found that the discharge capacity of the material increases with the decrease of particle size at -20 ° C, because the diffusion distance of lithium ions is shortened, making the process of lithium removal easier. Sun et al. Showed that the discharge performance of LiFePO4 decreased significantly with the decrease of temperature, and the materials with small particle size had higher capacity and discharge platform.
2、 Electrolyte
Electrolyte asLithium ion batteryIt not only determines the migration rate of Li in the liquid phase, but also participates in the formation of SEI film, which plays a key role in the performance of SEI film. At low temperature, the viscosity of electrolyte increases, the conductivity decreases, the impedance of SEI film increases, and the compatibility with anode and cathode materials becomes poor, which greatly worsens the energy density and cycle performance of the battery.
At present, there are two ways to improve low temperature performance through electrolyte:
(1) The low temperature conductivity of electrolyte can be improved by optimizing the composition of solvent and using new electrolyte salt;
(2) New additives are used to improve the properties of SEI film, which is conducive to the conduction of Li at low temperature.
1 optimize solvent composition
The low-temperature performance of electrolyte is mainly determined by its low-temperature CO melting point. If the melting point is too high, the electrolyte is easy to crystallize and precipitate at low temperature, which seriously affects the conductivity of electrolyte. Vinyl carbonate (EC) is the main solvent component of the electrolyte, but its melting point is 36 ° C. at low temperature, its solubility in the electrolyte decreases or even precipitates, which has a great impact on the low-temperature performance of the battery. By adding components with low melting point and low viscosity and reducing the content of solvent EC, the viscosity and co melting point of electrolyte at low temperature can be effectively reduced and the conductivity of electrolyte can be improved.
Kasprzyk et al. Obtained amorphous electrolyte by mixing EC and poly (ethylene glycol) dimethyl ether, and only a glass transition temperature point appeared near -90 ° C. This amorphous electrolyte greatly improved the performance of the electrolyte at low temperature; At -60 ° C, its conductivity can still reach 0.014ms · cm-1, which provides a good solution for the use of lithium-ion batteries at extremely low temperatures.
Chain carboxylate solvents have low melting point and viscosity, and their dielectric constant is moderate, which has a good effect on the low-temperature performance of the electrolyte. Dong et al. Used ethyl acetate (EA) as the cosolvent and lithium imine trifluoromethylsulfonate as the electrolyte salt. The theoretical melting point of the electrolyte reached -91 ° C and the boiling point reached 81 ° C. The results show that even at the extreme low temperature of -70 ° C, the ionic conductivity of the electrolyte still reaches 0.2ms · cm-1. Combined with the organic electrode as the positive electrode and the polyimide derived from 1,4,5,8-naphthalene anhydride as the negative electrode, the battery still has 70% of the capacity at room temperature at -70 ° C.
Smart et al. Have done a lot of research on chain carboxylates as electrolyte cosolvent to improve the low temperature performance of batteries. The research shows that using ethyl acetate, ethyl propionate, methyl acetate and methyl butyrate as the electrolyte cosolvent is conducive to the improvement of the low-temperature conductivity of the electrolyte and greatly improves the low-temperature performance of the battery.
2 new electrolyte salt
Electrolyte salt is one of the important components of electrolyte, and it is also the key factor to obtain excellent low-temperature performance. At present, the commercial electrolyte salt is lithium hexafluorophosphate, and the impedance of SEI membrane formed is large, resulting in its poor low-temperature performance. The development of new lithium salt is imminent. Lithium tetrafluoroborate has small anion radius, easy association, lower conductivity than LiPF6, but low charge transfer impedance at low temperature. As an electrolyte salt, it has good low temperature performance.
Zhang et al. Used linio2/ graphite as electrode materials, and found that the conductivity of LiBF4 was lower than that of LiPF6 at low temperature, but its capacity at -30 ° C was 86% of that at room temperature, while the capacity of LiPF6 based electrolyte was only 72% of that at room temperature. This was because the charge transfer impedance of LiBF4 based electrolyte was small and the polarization at low temperature was small, so the low temperature performance of the battery was better. However, LiBF4 based electrolyte cannot form a stable SEI film at the electrode interface, resulting in serious capacity attenuation.
Lithium difluoroacetate borate (LiODFB), as the electrolyte of lithium salt, has high conductivity under high and low temperature conditions, which makes lithium-ion batteries show excellent electrochemical performance in a wide temperature range. Li et al. Found that liodfb/libf4-ec/dms/emc electrolyte has good low-temperature performance at low temperature. The test shows that the capacity retention rate of graphite /li button battery at low temperature -20 ° C, 0.5c cycle for 20 weeks is: liodfb/libf4ec/dms/emc (53.88%) > lipf6ec/dec/dmc/emc (25.72%), the capacity retention rate of the former is much higher than the latter, and this electrolyte has a good application prospect in low-temperature environment.
As a new lithium salt, LiTFSI has high thermal stability, low association degree of cation and anion, and high solubility and dissociation degree in carbonate system. At low temperature, the high conductivity and low charge transfer impedance of lifsi electrolyte ensure its low temperature performance. Mandal et al. Used LiTFSI as the lithium salt and ec/dmc/emc/pc (mass ratio 15:37:38:10) as the basic solvent. The resulting electrolyte still has a high conductivity of 2ms · cm-1 at -40 ° C.
3 additives
SEI film has a very important influence on the low-temperature performance of the battery. It is an ionic conductor and electronic insulator, and a channel for Li to reach the electrode surface from the liquid phase. At low temperature, the impedance of SEI film increases, and the diffusion rate of Li in SEI film decreases sharply, which deepens the degree of charge accumulation on the electrode surface, resulting in the decline of lithium intercalation ability of graphite and the enhancement of polarization. By optimizing the composition and film forming conditions of SEI film, improving the ionic conductivity of SEI film at low temperature is conducive to the improvement of low temperature performance of battery. Therefore, the development of film forming additives with excellent low temperature performance is a research hotspot at present.
Liu et al. Studied the effect of FEC as an electrolyte additive on the low-temperature performance of the battery. The results showed that at -20 ° C, the capacity of the electrolyte added with 2 ° C increased by 50% compared with the basic electrolyte at -20 ° C for the first discharge, and the charging platform decreased by 0.2V. XPS test shows that the content of LIF in the SEI film formed by adding FEC electrolyte is higher than that of the SEI film formed by adding FEC electrolyte, which is conducive to the reduction of the impedance of the SEI film at low temperature, thereby improving the low temperature performance of the battery.
Yang et al. Found that adding lipo2f2 can significantly improve the low-temperature performance of lini0.5co0.2mn0.3o2/ graphite flexible battery. The capacity retention rate of the battery containing lipo2f2 electrolyte after 100 cycles at low temperature 0 ° C and -20 ° C is 96.7% and 91% respectively, while the capacity retention rate of the basic electrolyte after 100 cycles is only 20.1% and 16.0%. EIS tests were carried out on lini0.5co0.2mn0.3o2/li, full cell and graphite /li half cell. The results showed that the addition of lipo2f2 could significantly reduce the SEI film impedance and charge transfer impedance of graphite negative electrode, and reduce the polarization at low temperature.
Liao et al. Showed that the addition of BS (butyl Sultane, BS) in electrolyte is conducive to the improvement of battery discharge capacity and magnification performance at low temperature. They used EIS, XPS and other means to deeply discuss the mechanism of BS. At -20 ° C, the impedance rsei and RCT decreased from 4094 Ω and 8553 Ω to 3631 Ω and 3301 Ω respectively after the addition of BS, indicating that the addition of BS improved the charge transfer rate of lithium ions and greatly reduced the polarization at low temperature. XPS test shows that BS is conducive to the formation of SEI film, which can form sulfur compounds with low impedance, reduce the content of Li2CO3 in SEI film, reduce the impedance of SEI film, and improve the stability of SEI film.
In conclusion, the conductivity and film-forming impedance of electrolyte have an important impact on the low-temperature performance of lithium-ion batteries. For low-temperature electrolyte, it should be optimized from three aspects: electrolyte solvent system, lithium salt and additives. For the electrolyte solvent, the solvent system with low melting point, low viscosity and high dielectric constant should be selected. The linear carboxylate solvent has excellent low-temperature performance, but it has a great impact on the cycle performance. It needs to match the cyclic carbonate with high dielectric constant, such as EC and PC; For lithium salts and additives, the migration rate of lithium ions should be increased mainly by reducing the film-forming impedance In addition, properly increasing the concentration of lithium salt at low temperature can improve the conductivity of electrolyte and low temperature performance.
3、 Negative electrode material
The deterioration of diffusion kinetic conditions of lithium ion in carbon anode materials is the limitationLithium ion batteryThe main reason for the low temperature performance is that the electrochemical polarization of the negative electrode is significantly intensified during the charging process, which easily leads to the precipitation of metal lithium on the surface of the negative electrode.
Luders et al. Showed that at -20 ° C, the precipitation of lithium metal will be significantly increased if the charging ratio exceeds c/2. At c/2, the lithium precipitation on the negative surface is about 5.5% of the whole charging capacity, but at 1C, it will reach 9%. The precipitated lithium metal may further develop and eventually become lithium dendrites. Therefore, when the battery must be charged at a low temperature, it is necessary to choose a small current to charge the lithium-ion battery as much as possible, and fully put the lithium-ion battery aside after charging, so as to ensure that the metal lithium separated from the negative electrode can react with graphite and re embed into the graphite negative electrode.
Zinth et al. Used neutron diffraction and other means to study the lithium evolution behavior of nmc111/ graphite 18650 lithium ion battery at low temperature -20 ° C. the battery is charged and discharged as shown in Figure 2. Figure 3 shows the comparison of the phase changes of graphite cathode when charging at c/30 and c/5 rates respectively.
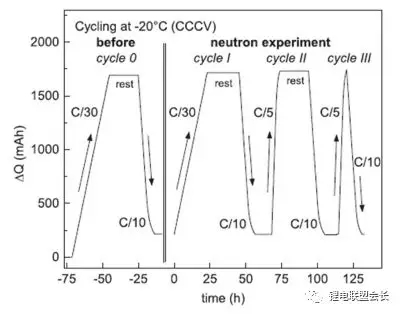
Fig. 2 charge and discharge process of neutron diffraction experiment at low temperature -20 ° C Δ Relationship between Q and time
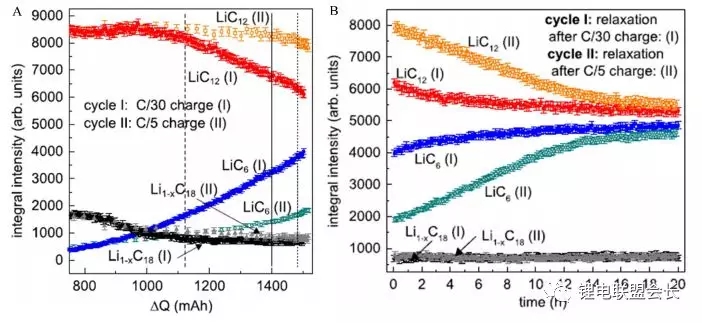
Fig. 3 Comparison of negative electrode phase changes after charging (a) at different rates and shelving for 20h (b)
It can be seen from the figure that for the two different charging rates, the lithium poor phase li1-xc18 is very similar, and the difference is mainly reflected in the two phases lic12 and lic6. At the initial stage of charging, the phase change trend in the negative electrode is relatively close under the two charging rates. For the phase lic12, when the charging capacity reaches 95mah, the change trend begins to appear different, and when it reaches 1100mah, The lic12 phase at the two rates began to show a significant difference. When charging at a low rate of c/30, the lic12 phase decreased very fast, but the lic12 phase at a low rate of c/5 decreased much more slowly, that is to say, due to the poor lithium intercalation kinetic conditions of the negative electrode at low temperature, the further lithium intercalation of lic12 into lic6 phase decreased. Correspondingly, the lic6 phase increased very fast at a low rate of c/30, However, at c/5 rate, it is much slower, which indicates that at c/5 rate, less Li is embedded in the crystal structure of graphite, but the charging capacity of the battery at c/5 charging rate is a little higher than that at c/30 charging rate. The extra Li that is not embedded in the graphite cathode is likely to precipitate on the graphite surface in the form of metal lithium, which is also confirmed by the static process after charging.
Zhang et al. Measured the trend of impedance parameters re, RF and RCT of graphite /li half cell with temperature by EIS method, and found that the three increased with the decrease of temperature, in which the growth rate of RE and RF was roughly the same, while the growth rate of RCT was faster. When the temperature decreased to -20 ° C, RCT had become the main component of the total impedance of the battery, which showed that the deterioration of electrochemical reaction kinetic conditions was the main factor causing the deterioration of low-temperature performance.
The selection of appropriate anode materials is the key factor to improve the low-temperature performance of the battery. At present, the low-temperature performance is optimized mainly through anode surface treatment, surface coating, doping to increase the layer spacing, and controlling the particle size.
1 surface treatment
Surface treatment includes surface oxidation and fluorination. Surface treatment can reduce the active sites on the graphite surface, reduce irreversible capacity loss, and generate more micro nano structured pores, which is conducive to Li transmission and reduce impedance.
Zhang Lijin et al. After oxidation micro diffusion treatment, the average grain size of graphite decreases, the amount of lithium ions embedded on the surface and edge of the carbon layer increases, and the nano pore structure introduced on the surface of graphite further increases the storage space of lithium ions. Wu et al. Fluorinated natural graphite with 5AT% fluorine gas at 550 ° C, which greatly improved the electrochemical performance and cycle performance of the treated material.
2 surface coating
Surface coating such as carbon coating and metal coating can not only avoid the direct contact between the negative electrode and the electrolyte, improve the compatibility between the electrolyte and the negative electrode, but also increase the conductivity of graphite, provide more embedded lithium sites, and reduce the irreversible capacity. In addition, the layer spacing of soft carbon or hard carbon materials is larger than that of graphite. Coating a layer of soft carbon or hard carbon materials on the negative electrode is conducive to the diffusion of lithium ions, reduce the SEI film impedance, and improve the low-temperature performance of the battery. The surface coating of a small amount of Ag improves the conductivity of the cathode material, making it have excellent electrochemical performance at low temperature.
The fe/fe3c-cnf composite developed by Li et al. Has good low temperature performance, and still maintains a capacity of 250mah · g-1 after 55 cycles at -5 ° C. Ohta et al. Studied the effects of different anode materials on the performance of lithium-ion batteries, and found that the irreversible capacity of both carbon coated artificial graphite and natural graphite was significantly lower than that of uncoated. At the same time, carbon coated graphite anode can effectively improve the low-temperature performance of the battery. The discharge capacity retention rate of 5% coated graphite at -5 ° C is 90% of that at room temperature. Nobili et al. Used graphite coated with metal tin as the negative electrode material. At -20 ° C, the SEI film impedance and charge transfer impedance were reduced by 3 times and 10 times respectively compared with the uncoated material, which showed that the coating of tin could reduce the polarization of the battery at low temperature, thereby improving the low temperature performance of the battery.
3 increase the graphite layer spacing
The layer spacing of graphite cathode is small, and the diffusion rate of lithium ions between graphite layers decreases at low temperature, resulting in increased polarization. The introduction of B, N, s, K and other elements during the preparation of graphite can modify the structure of graphite, increase the layer spacing of graphite and improve its lithium removal / intercalation ability. The atomic radius of P (0.106pm) is larger than that of C (0.077pm). Doping p can increase the layer spacing of graphite and enhance the diffusion ability of lithium ions, At the same time, it is possible to increase the content of graphite microcrystals in carbon materials. The insertion compound kc8 will be formed when k is introduced into the carbon material. When potassium is removed, the layer spacing of the carbon material will increase, which is conducive to the rapid insertion of lithium, thereby improving the low-temperature performance of the battery.
4 control the particle size of negative electrode
Huang et al. Studied the effect of negative particle size on low temperature performance and found that the average particle size was 6 μ M and 25 μ M coke cathode has the same reversible charge discharge capacity at room temperature, while at -30 ° C, the particle size is 25 μ The coke electrode of M can only release 10% of the capacity at room temperature, and the particle size is 6 μ M coke electrode can release 61% of room temperature capacity.
From this experimental result, it can be concluded that the larger the particle size of the negative electrode, the longer the lithium ion diffusion path and the larger the diffusion impedance, resulting in the increase of concentration polarization and the deterioration of low-temperature performance. Therefore, appropriately reducing the particle size of the negative electrode material can effectively shorten the migration distance of lithium ions between the graphite layers, reduce the diffusion impedance, increase the electrolyte infiltration area, and then improve the low-temperature performance of the battery. In addition, the graphite anode granulated by small particle size single particle has high homogeneity, which can provide more lithium insertion sites, reduce polarization, and significantly improve the low-temperature performance of the battery.
4、 Conclusion
To sum up, the low temperature performance of lithium-ion batteries is a constraintlithium batteryKey factors of application, how to improveLithium batteryThe low temperature performance of the pool is still the hot and difficult point of current research.
The reaction process of the battery system mainly includes four steps: Li transport in the electrolyte, through the electrolyte / electrode interface facial mask, charge transfer and Li diffusion in the active substance body. At low temperature, the rate of each step decreases, which leads to the increase of impedance of each step, the intensification of electrode polarization, and the reduction of low-temperature discharge capacity and lithium evolution of the negative electrode.
In order to improve the low-temperature performance of lithium battery, the influence of positive electrode, negative electrode, electrolyte and other comprehensive factors in the battery should be comprehensively considered. By optimizing the composition of electrolyte solvent, additives and lithium salt, the conductivity of electrolyte should be improved and the film-forming impedance should be reduced at the same time; The positive and negative materials were modified by doping, coating, small granulation, etc., so as to optimize the material structure and reduce the interface impedance and the diffusion impedance of Li in the active material body. Through the overall optimization of the battery system, the polarization of lithium battery at low temperature is reduced, and the low temperature performance of the battery is further improved.
Article source:Lithium power grid
0755-89480969
info@powercome.hk
B1202, building 1, Mogen Fashion Industrial Park, No. 10, shilongzi Road, Xinshi community, Dalang street, Longhua District, Shenzhen
www.powercome.hk