2019-11-28
Recently, Professor Pan Feng and Associate Professor Zheng Jiaxin from the school of new materials, Shenzhen Graduate School, Peking University, jointly wrote an opinion article on the National Science Review (NSR) "structure units' as materials genes in category materials for literature on batteries"lithium batteryHow the structural elements in cathode materials determine their internal physical and chemical properties (conductivity, ion migration, structural stability, thermal stability and charge transfer properties) plays the role of "material gene".
In view of the rich and wonderful content of the article, OFweekLithium batteryOn the basis of loyalty to the original text, the website translated the original text into the following:
The basic structural units of crystals are lattice atoms and their coordination environment. They are arranged periodically in specific combinations (such as space groups) to form crystals. Generally speaking, the bonding interaction and electronic structure in structural units determine the inherent physical and chemical properties of crystals, similar to the key role of genes in life.
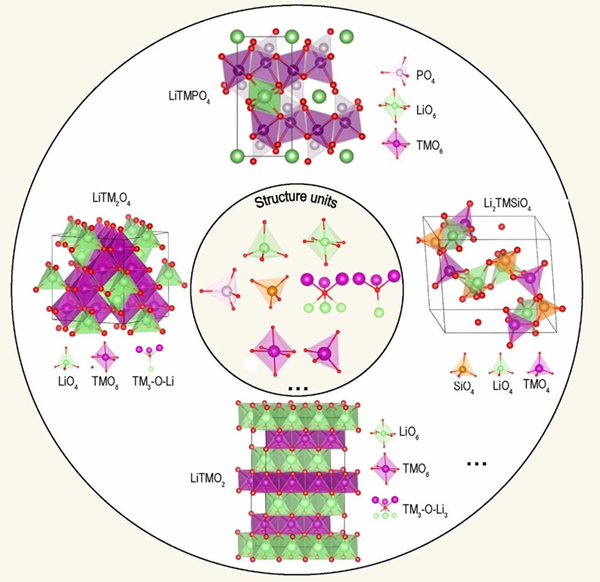
Structural elements in representative cathode materials of lithium batteries. The small circle in the figure shows the structural elements, and the outer circle is the cathode material of lithium battery formed by the arrangement and combination of these structural elements. Source: Thesis
Lithium ion batteryAll cathode materials are composed of lithium, transition metals and negative ion structural units. For example, in the olive polyanion LiFePO4 framework, feo6 octahedron of Fe-O coordination bond forms a two-dimensional network on the bc plane through shared o-angle connection, PO4 tetrahedron has strong P-O coordination bond, covalent bond is physically separated, connecting the attachment feo6 plane and lio6 plane, and octahedron of lithium ion band forms a one-dimensional b-axis channel through shared o-angle connection for lithium ion diffusion. Studying the physical and chemical properties of cathode materials from the perspective of structural units will lead to a better and deeper understanding of the intrinsic electrochemical properties and provide guidance for the rational design of cathode materials at the high-performance atomic level, which has attracted more and more attention. Advanced experimental characterizations commonly used to reveal the structure property relationship include time-resolved X-ray diffraction, neutron powder diffraction, X-ray absorption, mass spectrometry, and high-resolution transmission electron microscopy. However, these experimental tools are still difficult to observe structural units directly. The study of the relationship between structural units and electrochemical properties mainly depends on theoretical simulation, such as ab initio calculation, ab initio molecular dynamics simulation, and tight combination methods.
Almost all cathode materials of lithium batteries are semiconductors, and their electronic conductivity depends on the d-orbital electrons of transition metals (TM). For example, the diffusion of electrons inside and outside LiFePO4 depends on the feo6 2D framework. The coordination environment of TM in TM unit (tmox) determines the energy and spatial distribution of d-orbital electrons, which directly affects the conductivity of electrons. For example, the d electrons of CO in coo6 in LiCoO2 will be delocalized during the process of iron removal, and the semiconductor phase will be transformed into a metal phase. In contrast, the d electrons of Fe in LiFePO4 and Li2FeSiO4 become more localized in the process of iron removal, and the localized electronic states will be coupled with the local atomic lattice deformation to form polarons, which is not conducive to the conductivity of electrons and ultimately affect the charge and discharge performance. The transport of lithium ions in cathode materials is also closely related to the structural units and arrangement of cathode materials. For example, Li ions in layered linixmnycozo2 (NMC) diffuse from one octahedral site (lio6) to another octahedral site, which is controlled by one or two octahedral coordinated TM ions. Therefore, the valence state of TM ions and the size of tmo6 and lio6 / lio4 will directly affect the migration of Li ions. Using the above structural unit model, The predicted trend of lithium ion diffusion coefficient changing with nickel content in NMC materials is verified by experimental measurement results.
The structural stability of cathode materials is related to structural units. For example, in the crystal structure of LiFePO4, the tetrahedron of PO4 with strong P-O covalence is used as the node connecting the feo6 surface of the appendage, which establishes excellent structural stability during the electrochemical cycle. In the process of hydrogen sulfide removal, the distortion of tmo6 in layered cathode material is the cause of disordered crystal structure and phase transition. In addition to structural stability, the thermal stability of cathode materials also depends on structural units. Using ab initio calculation, we previously proved that the thermal stability of lattice oxygen in layered cathode material (tm3-o-lix) determines the thermal stability of [8], which can be adjusted by changing the type of TM and the number of lithium ions and adjusting their position in tm3-o-lix. For example, in nickel rich NMC materials, the exchange of Ni and Li will form a 180 ° ni-o-ni super exchange chain in tm3-o-lix. Spin parallel Ni form between o ions σ- Bonding Ni π - bonding form of reverse rotation between nickel ion and O ion. This will improve the thermal stability of nickel rich NMC materials. The theoretical prediction of the thermal stability of NMC materials based on the above tm3-o-lix unit model is consistent with our previous experimental measurement results (in-situ time-resolved X-ray diffraction and thermogravimetric analysis). Combining our recent experimental work with theoretical calculations, we report that replacing sbo6 with 1 / 3 NiO6 in nano-2 and constructing a highly ordered (NiO6) 6-ring structure in TM layer will enhance structural stability and thermal stability.
Most cathode materials are so-called charge transfer materials, and the charge transfer process is related to the capacity and voltage of cathode materials. After lithium ion extraction, titanium oxide ions are considered to be the only electrochemically active source of charge compensated electrons in the inserted cathode. The energy required to oxidize TMN + to TM (n + 1) + depends on the energy level of the TM d orbital in the TM unit. For example, the energy required to remove an electron from the t2g level of octahedral coordinated Mn4 + is significantly greater than that required to remove an electron from the t2g level of tetrahedral coordinated Mn4 +. Recent observations have questioned the above image, suggesting that oxygen ions may also participate in redox reactions in oxide cathodes. Since transition metal ligand hybridization contributes to the highest valence band, it is not surprising that the oxygen o-2p state close to the Fermi level promotes reversible oxygen redox. Ceder et al. Used the mixed functional hse06 (SIE can be modified to a certain extent) to calculate the DFT, and believed that the oxygen redox in the lithium rich layered cathode material was due to the excess lithium in the oxygen structural unit (tm3-o-li3), which promoted the formation of li-o-li o-2p non bonded orbitals at the Fermi level. They further reported that in lithium rich layered cathode materials, replacing o with F can reduce Ni3 + / Ni4 + to Ni2 + state, which not only increases the Ni redox pool, but also prevents the loss of oxygen caused by excessive redox of compounds. A recent important theoretical work has proved that in lithium rich transition metal oxides, the reversibility of anion capacity is limited to the critical number of O pores per oxygen in the tmo6 unit.
It should be noted that there is also synergy among units. For example, at a certain temperature and pressure, the storage chemical potential of lithium ions in electrode materials is determined by the synergistic interaction between the redox couple of TM unit and the ionic bond of lithium ion unit. As mentioned earlier, the migration of lithium ions is also directly affected by the synergistic effect of TM (tmox) and Li ions (liox).
To sum up, the structural units in cathode materials can be regarded as material genes, which determine the electronic conductivity, lithium ion transport, structure and thermal stability, and charge transfer characteristics. The understanding of the relationship between structural units and physicochemical properties has promoted the transformation of the traditional electrochemical interpretation of LIBS cathode materials from volume / surface based interpretation to more local chemical combination interpretation. This provides a simple and direct guidance for improving the electrochemical performance of cathode materials or designing high-performance cathode materials by regulating material genes (such as genetic engineering in Biology). One way is to directly adjust material genes. For example, replacing TM and o with other elements in the tmox unit, or destroying the symmetry of tmox through deformation or pressure, will be an effective method to adjust the electronic structure of cathode materials. Lithium ion diffusion can be adjusted by replacing TM of different valence metals in tmox unit and adjusting the size and arrangement of tmox unit and liox unit. Another method is to look for possible high-performance structures from material genes. First, you must select a group of structural units and select the number of times each structural unit appears in a single repeating unit. Next, evolutionary algorithm and other methods are used to search all possible permutations of structural units, and the number of candidate structures is reduced by deleting duplicates of the same permutation. Finally, the relative stability of the remaining candidate structures is quantitatively evaluated by ab initio calculation, and these final theoretically predicted stable structures are ranked according to appropriate selection criteria (such as energy density, stability and rate performance), so as to provide the most promising candidate structures for experimental synthesis.
Article source:OFweek lithium grid
0755-89480969
info@powercome.hk
B1202, building 1, Mogen Fashion Industrial Park, No. 10, shilongzi Road, Xinshi community, Dalang street, Longhua District, Shenzhen
www.powercome.hk